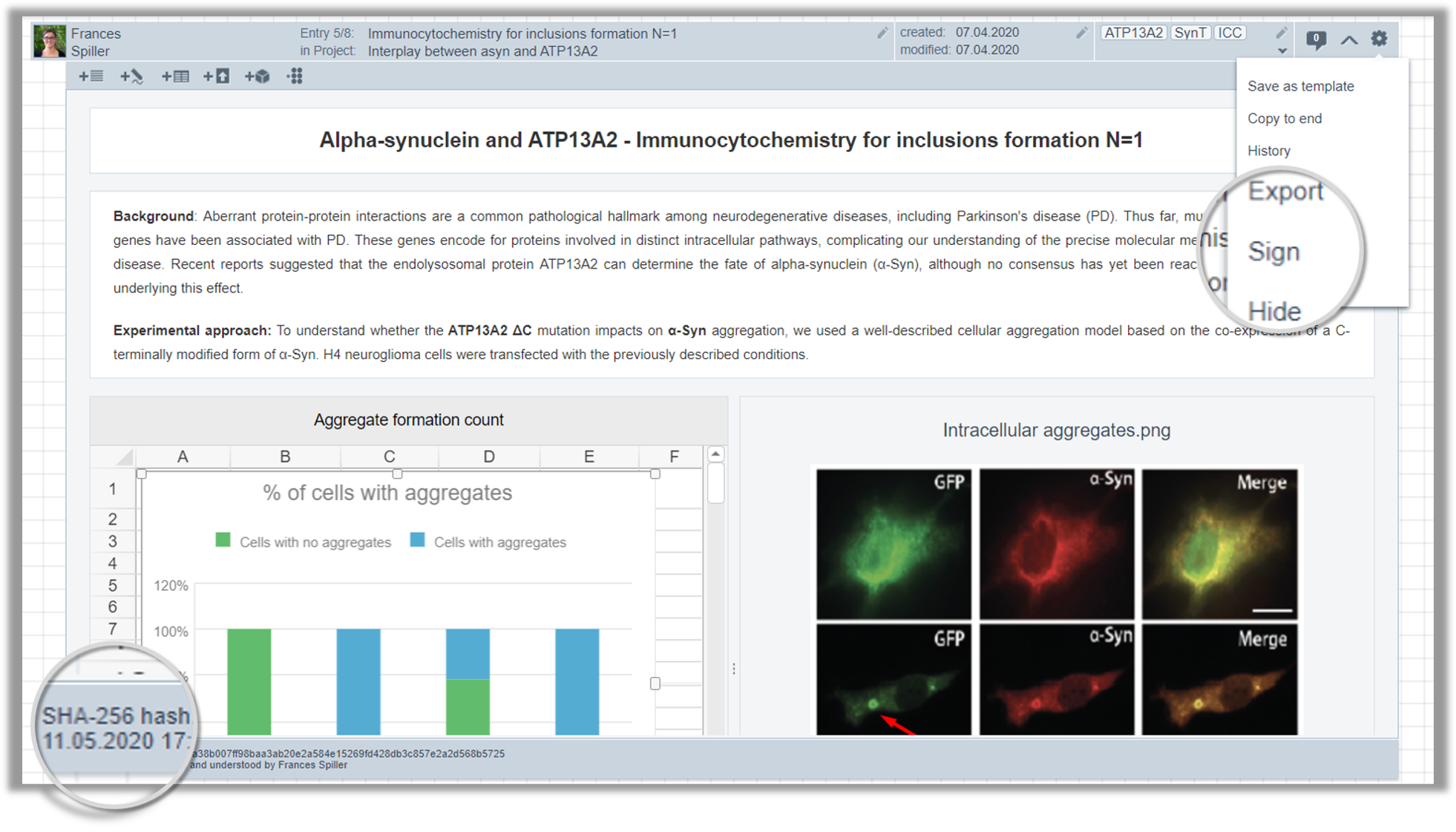
Data integrity and compliance are the most important topics in scientific research. Labfolder’s Sign&Witness and Signature Workflows are designed to streamline the process of signing and verifying entries within collaborative projects using FDA-compliant digital signatures.
In this webinar session, you will learn about their differences and benefits, helping you to assess which tool would be most suitable for your work. Furthermore, we will explain in detail how Signature Workflows can be customized to meet your needs in a verification process.
This is especially beneficial for industry clients or institutes that follow complex validation processes.
Dr. Frances Spiller – Customer Success Specialist at Labforward

Before joining Labforward, Frances obtained a PhD in Molecular Biology from the University of Edinburgh. Her long research experience makes her a valuable member of the support team, where she facilitates customer communications.
No webinars planned just now!